Preclinical studies look at efficacy
The search for a new medical product begins with a precise analysis of the disease process. At which point can active ingredients intervene and positively influence the process of a disease? If suitable active ingredients and therapeutic approaches are already known, the next important step is to optimize the substances for further studies.
The aim of the preclinical phase is to identify substances that show a therapeutic effect and are suitable for further testing and the subsequent clinical phases.
During the preclinical study, we first test new active ingredients or combinations of active ingredients in vivo and then in animal models. Before a substance can be used in a clinical study, its efficacy must be proven. If the substance proves ineffective, it has little chance of further development. If the preclinical studies are successfully completed, the clinical trial can be prepared. If desired, we work under GLP conditions and can draw on many years of experience in our large animal laboratory. With our services, we offer targeted support for optimal product development and successful testing and approval.
Services
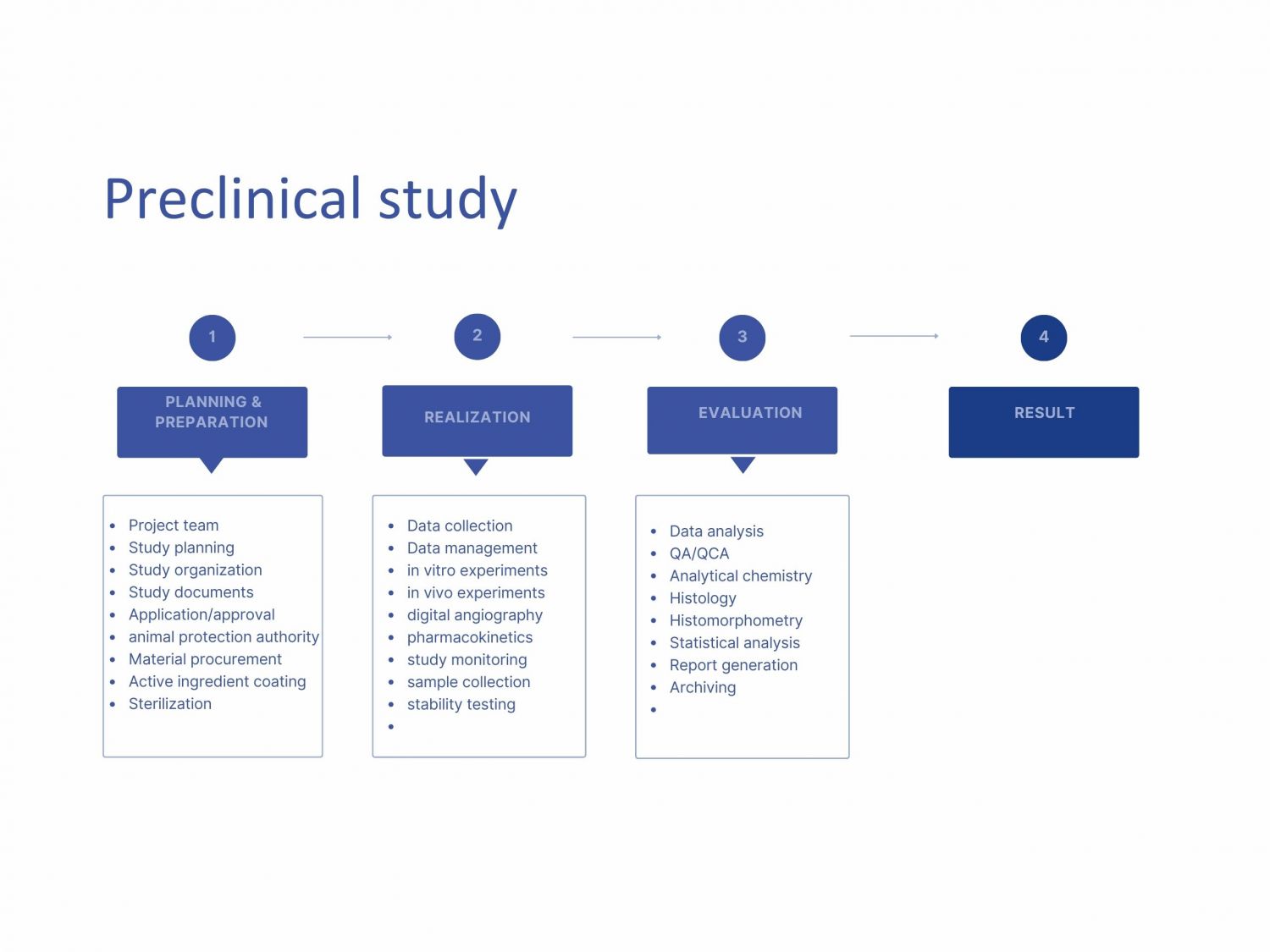
- Planning, organizing and monitoring projects on behalf of the contractual partners
- Design of study protocols, documentation forms, etc.
- Analytical chemistry, formulation of drugs, stability testing, sterilization
- In-vitro and in-vivo testing of relevant pharmaceutical properties, pharmacokinetics, efficacy and tolerability
- Special laboratory for digital angiography
- Applications for approval from ethics commissions, animal protection authorities, etc.
Evaluate the efficacy in the preclinical phase