Clinical studies in medical device development
Clinical studies are always an important part of medical device development in order to evaluate and substantiate the safety, efficacy and performance of medical devices with data. Our clinical studies are conducted by an experienced clinical trials team. We work closely with clinics, medical professionals and statisticians to ensure that the studies meet the highest scientific standards.
Services
- Complete project coordination
- Planning of the study design and the entire study procedures
- Selection of qualified trial centers
- Drafting of contracts with the trial centers
- Preparation of the necessary study documents (including study protocol, patient information and consent, monitoring plan, ISF, TMF)
- Development of CRF: paper or electronic
- Submission of the documents to the relevant authorities and obtaining approvals or ethics votes
- Communication with the authorities and ethics committees
- Monitoring: selection visits, initiation visits, routine visits, final visits
- Risk management
- (S)AE Management
- Device management
- Site management
- Support for inspections and audits
- Data and query management
- Organization and coordination of statistical evaluations
- Preparation of study reports
- Preparation of manuscripts for publications
- Archiving
InnoRa CRO GmbH - Your partner
for clinical trials
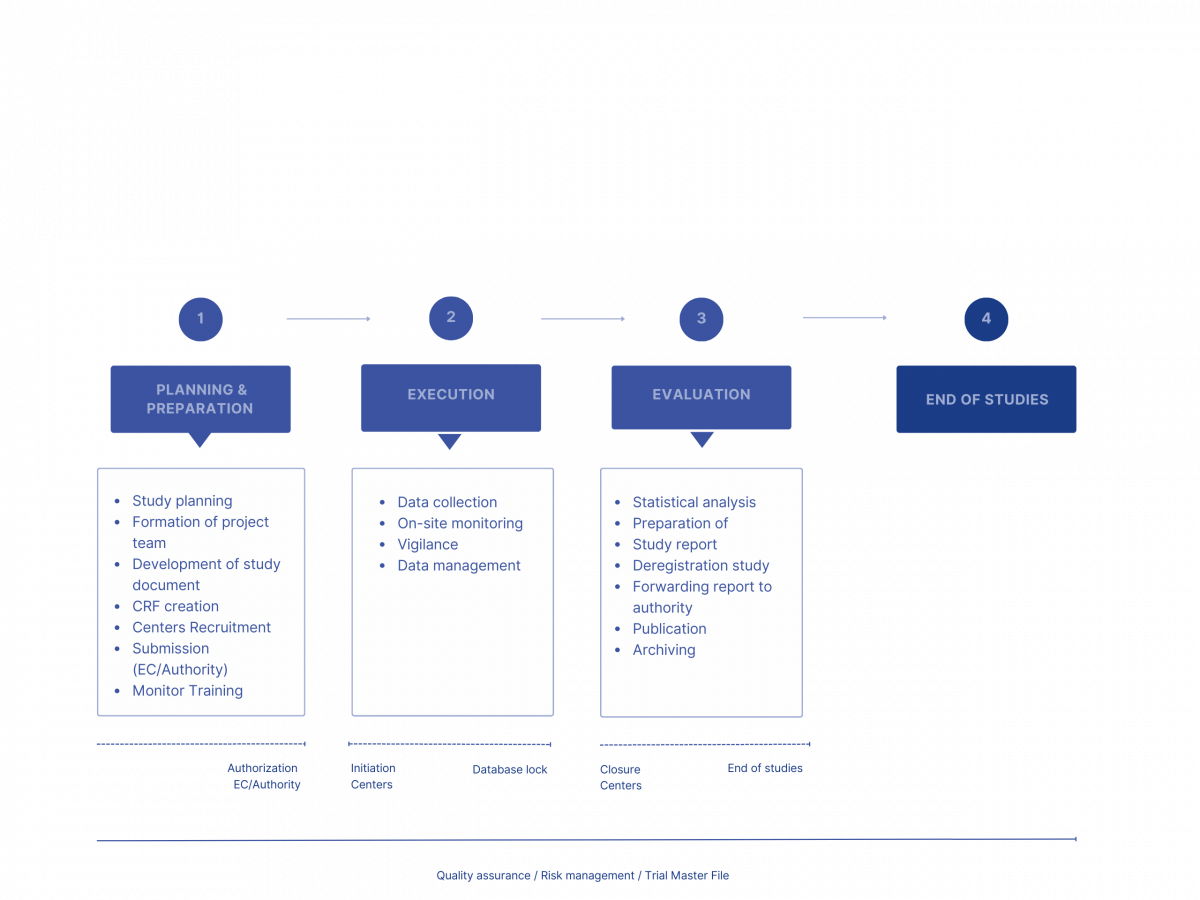
Process steps in clinical trials
At InnoRa CRO GmbH, we accompany you step by step through all phases: from the conception of your study idea and the development of the study design to the approval process with the responsible authorities and ethics committees to the comprehensive evaluation and publication of the results. We take over the administrative handling of your study and guarantee valid data quality through qualified monitoring.
Scientific work - Publications
On our website you will find published information on our clinical studies, including study design, participating centers, inclusion criteria and results.